News
Excellamol has received a promising review from the BTR-NTA Committee! (Aug. 12, 2025)
Excellamol was honored to be selected for the BTR-NTA program (www.tessajowellbraincancermission.org/strategic-programmes/btr-nta), held on May 21, 2025, in Edinburgh, Scotland. The company showcased its Self-Depot™ OncoPDC platform and XM161-SN38, an FDA Orphan Drug Designated therapy for brain cancers, and engaged with eighteen leading experts across the therapeutic development pathway. Feedback on preclinical progress, manufacturing, regulatory strategy, biomarkers, and trial design will help guide Excellamol’s next steps in advancing therapies for patients with brain cancer.
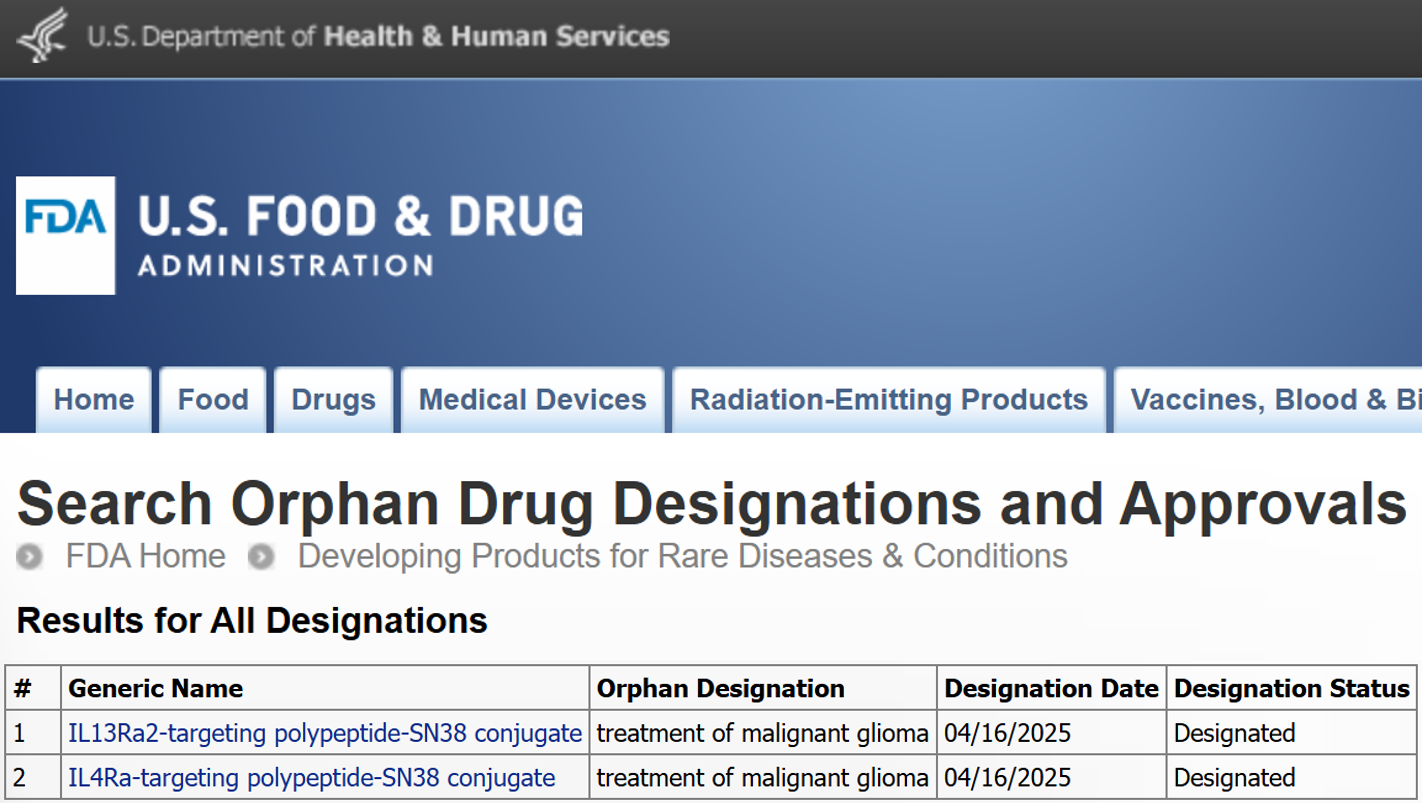
U.S. FDA grants Orphan Drug Designation to Excellamol’s two leading OncoPDCs for the treatment of malignant glioma! (Apr. 16, 2025)
The FDA’s Orphan Drug Designation for XM147-SN38 and XM161-SN38 in malignant glioma marks an important regulatory milestone and underscores the strong scientific foundation of Self-Depot™ OncoPDC program. Advancing innovative therapies for patients with rare and hard-to-treat solid tumors remains central to Excellamol’s mission. Supported by data expected from our upcoming Phase 1 study, we will assess the most effective path forward for clinical development, including potential expansion into rare pediatric brain tumor indications.